Understanding Pregnancy Skin from a Formulator’s Perspective
Formulators are no strangers to the complexity of skin physiology—but pregnancy introduces a new set of variables that are both dynamic and deeply consequential. From the moment conception occurs, a woman’s body undergoes hormonal, vascular, and structural transformations that affect not just the skin’s appearance but also its function, resilience, and sensitivity. What once worked may now irritate. What once seemed unnecessary becomes essential.
As demand grows for safe and effective cosmetic solutions that cater to pregnancy and postpartum consumers, formulators must navigate a heightened landscape of risk, compliance, and trust. The modern maternal consumer is informed and cautious, scrutinizing INCI lists with the same intensity as nutrition labels. But the challenge isn’t just about what to avoid—it’s about understanding what the skin needs during each stage of pregnancy and how to deliver that safely and effectively.
Navigating Shifting Skin Needs: From First Trimester to Postpartum
Each trimester of pregnancy brings its own pattern of skin disruption. In the first trimester, hormonal fluctuations—particularly rising progesterone—can trigger acne, sebaceous hyperactivity, and increased sensitivity. Formulators must balance gentle cleansing and barrier reinforcement without using ingredients contraindicated for systemic absorption.
By the second trimester, increased vascularization and estrogen levels often lead to pigment disorders like melasma. The skin becomes more reactive to UV exposure, and the melanocyte-stimulating hormone (MSH) peaks. Melasma, colloquially known as the “mask of pregnancy,” demands safe brightening strategies that avoid hydroquinone and retinoids—both of which are off-limits during pregnancy.
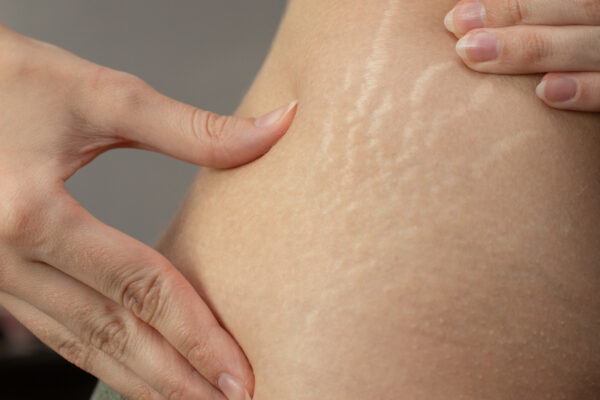
In the third trimester, as the body prepares for delivery, the skin is under mechanical stress. Stretch marks (striae gravidarum) begin to form due to rapid collagen disruption and elastin fiber thinning. It’s a window where support for dermal remodeling—within strict safety thresholds—becomes critical. Concurrently, TEWL may increase, demanding occlusive and humectant-rich formulations.
Postpartum recovery, particularly for breastfeeding mothers, introduces another challenge. Barrier repair, elasticity recovery, and hydration must be addressed while ensuring that ingredients are non-toxic if transferred through nursing. Products applied near the chest must be free of strong preservatives, fragrances, or penetration enhancers that could impact the baby through oral mucosal contact.
A Delicate Balance: Why Maternal Skin Requires a Different Approach
Pregnancy-safe formulation is not simply “clean beauty plus a few edits.” It’s a rigorous exercise in toxicological risk assessment, sensitivity analysis, and targeted efficacy. The risk of systemic absorption, placental transfer, or ingestion by a breastfeeding infant limits the available toolbox significantly. Retinoids, high-percentage AHAs, BHA (salicylic acid), formaldehyde donors, essential oils, certain chemical UV filters (like oxybenzone), and most skin-lightening agents must be avoided entirely.
But it’s not just about exclusions. The real challenge lies in delivering clinical-grade results with a reduced set of allowable actives. For example, how do you address hyperpigmentation without tyrosinase inhibitors like hydroquinone or kojic acid? How do you soothe inflammation without steroids or conventional anti-inflammatory drugs? And how do you support collagen remodeling without triggering irritation from vitamin A derivatives?
Formulators must also consider ingredient traceability, allergenicity, and mucosal safety—especially for nipple balms or any product applied to the chest. All ingredients must meet stringent thresholds for ingestion safety or transdermal passivity, often borrowing standards from baby care, pharmaceutical-grade topicals, or food-grade excipients.
Inside the Pregnancy-Safe Toolbox: Building Maternal-Safe Systems That Perform
There is a misconception that safety means compromise—but when used correctly, certain categories of ingredients can deliver both peace of mind and performance.
For barrier support and hydration in all stages, humectants like glycerin, hyaluronic acid, and sodium PCA provide gentle, non-sensitizing moisture retention. Emollients such as squalane, sunflower seed oil, and sweet almond oil nourish without risk of irritation or systemic harm. For increased skin resilience, ceramides and panthenol support the lipid matrix and reduce TEWL.

When addressing pigment regulation, formulators should turn to niacinamide, azelaic acid, or tranexamic acid at low concentrations. These offer tyrosinase-modulating effects with low irritation risk and strong safety records in pregnancy. For skin soothing, colloidal oatmeal, aloe vera (decolorized, food-grade), and centella asiatica extracts offer anti-inflammatory benefits with minimal sensitization.

For stretch mark prevention and elasticity support, rosehip oil, peptides, and vitamin E derivatives (tocopherol) promote collagen health and skin suppleness. These ingredients can be integrated into body creams and oils, provided they avoid excessive penetration enhancers or sensitizing botanicals.

Postpartum nipple and baby-safe care should only include pharmaceutical-grade lanolin, coconut oil, beeswax, and non-preserved butters like shea or cocoa, as these pose minimal risk when ingested and provide barrier protection for healing skin.
Staying Within the Safety Lines
The most effective maternal products begin with a well-documented risk assessment. Safety dossiers should prioritize ingredient sources that have been validated for food, pharmaceutical, or pediatric use. INCI-level traceability, especially for natural extracts, must include residual solvent, heavy metal, and pesticide analyses.
Avoid overcomplicating the INCI list. Minimalist formulas are not only consumer-preferred but reduce the overall exposure burden. Fewer ingredients mean fewer unknowns in terms of allergic response or unintended synergies. Choose preservation systems with well-understood safety profiles, such as potassium sorbate, sodium benzoate, or lactic acid systems, when necessary.
If formulating for breastfeeding use, topical products that could contact the nipple should be fragrance-free, preservative-free (or self-preserving), and use edible-grade components. Even ingredients with otherwise stellar safety profiles—such as essential oils or plant acids—should be reconsidered due to their irritant potential to infants.
What Pregnancy-Safe Formulation Really Means
At its core, pregnancy-safe formulation is about more than just avoiding “bad” ingredients. It’s about understanding the biological context of the user—her skin, her body, her infant—and creating a formulation that is both responsive and respectful. The formulation must function within a narrower window of both physiological tolerance and ethical trust.
For formulators, this represents both a limitation and an invitation. A limitation in terms of ingredients, yes—but an invitation to innovate within a more meaningful design brief: safety, efficacy, and integrity. When done well, these products earn a level of consumer loyalty that transcends trends.
In an industry moving toward wellness, transparency, and holistic design, pregnancy and postpartum products are no longer a niche—they are a proving ground for responsible, performance-driven formulation.
Ready to formulate for mom and baby? Contact Deveraux Specialties to learn more about pregnancy- and postpartum-safe ingredients you can trust to meet both safety expectations and formulation versatility.
Featured Products
DL Goji Prebiotic
Glycyrrhetinic Acid & 18-b Glycyrrhetinic Acid Phytosome®
HydrActive™ Almond Oil
Resources
- Chan, M. et al. Use of Personal Care Products During Pregnancy and Birth Outcomes – A Pilot Study. Environmental Research. 2023. https://doi.org/10.1016/j.envres.2023.115583
- Biskanaki, F. et al. The Risk of Using Cosmetics and Cosmetic Procedures During Pregnancy. Applied Sciences. 2024. https://doi.org/10.3390/app14219885
- Lang, C. et al. Personal Care Product Use in Pregnancy and the Postpartum Period: Implications for Exposure Assessment. Int. J. Environ. Res. Public Health. 2016. https://doi.org/10.3390/ijerph13010105