Why Transcriptional “Noise” Is the New Aging Signal
Formulators have long mastered the levers of moisturization, barrier support, and antioxidant defense. Yet even impeccable bases can stall when the biology beneath the surface no longer “reads” its own instructions cleanly. As cells age, the mechanisms that control which genes are transcribed—and how precisely they’re transcribed—grow unstable. The result is transcriptomic “noise”: aberrant, cryptic transcription and splicing variants that yield truncated or dysfunctional proteins. Over time, that noise accumulates as visible fatigue in texture, elasticity, tone, and recovery. Recent reviews in mammalian systems tie this rise in cryptic transcripts to age-linked chromatin drift and epigenetic stress, offering a concrete, testable target for intervention.
The industry’s pivot from “anti-aging” to skin longevity aligns with this shift. Rather than pushing more ingredients at larger loads, the higher-order challenge is restoring transcriptional fidelity so that existing cellular programs can execute cleanly. In this context, Givaudan Active Beauty’s platform work on neuro-skin aging (sensory decline with age) and cryptic transcription reframes the brief for modern actives: protect neuronal-skin crosstalk, stabilize epigenetic control, and reduce erroneous transcripts that seed dysfunctional proteins.
From Elegant Textures to Faithful Transcripts
First, the biology: the primary hallmarks of aging—genomic instability, telomere attrition, epigenetic alterations, and loss of proteostasis—interlock to amplify transcriptional errors and protein dysfunction. When DNA repair (e.g., PARP1-mediated) is sluggish, when telomerase support is low, when DNMT3B-mediated methylation wanes, or when proteasome turnover slows, damaged templates and mis-made proteins accumulate. These are upstream failure points that conventional moisturizers or film formers can’t correct on their own.
Second, the formulation reality: raising active loads to chase instrumented outcomes often narrows pH windows, strains preservation, and destabilizes rheology—especially in fluid O/W and gel-cream architectures. Meanwhile, proteostasis declines with age, so the skin’s ability to clear malformed proteins is intrinsically lower in the target consumer. That means your formula has to do more with less burden: less thickening, fewer conflicts, and tighter doses that still move meaningful biomarkers.
Precision over Load: Givaudan’s Epigenetic-to-ECM Strategy for Neuro-Skin Longevity
Aging skin doesn’t just lose moisture; it loses instructional precision. When chromatin control slips and cryptic transcripts rise, even well-built formulas struggle to convert actives into reliable, instrumented gains. Givaudan Active Beauty’s longevity platform is designed specifically for this failure mode. It targets upstream governance—epigenetic tuning, transcriptional fidelity, and protein quality control—so downstream biomarkers (repair, elasticity, evenness) move in concert at dose-efficient levels. The result is a toolkit that aligns epigenome → transcriptome → proteome, giving formulators a practical way to build for neuronal comfort, barrier integrity, and visible resilience without overloading bases:
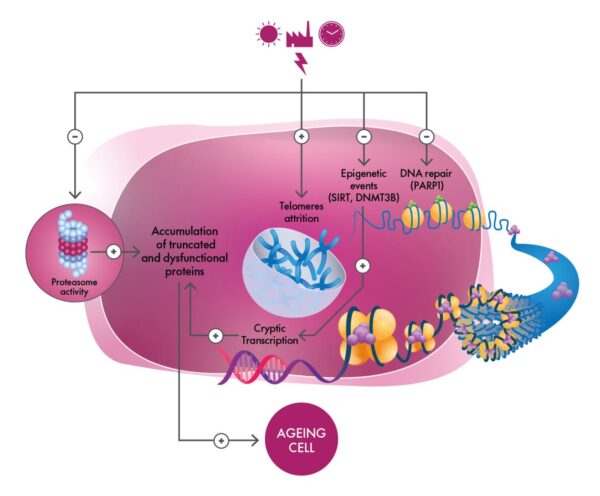
Mechanistically, the logic is coherent with the aging literature: sirtuins stabilize chromatin and coordinate telomerase; PARP1 powers base-excision repair; and proteasome activity preserves protein quality—while DNMT3B helps maintain methylation patterns that suppress cryptic promoters. When UltraReverse elevates these actors, it addresses epigenetic alterations, genomic instability, telomere attrition, and loss of proteostasis in one move.
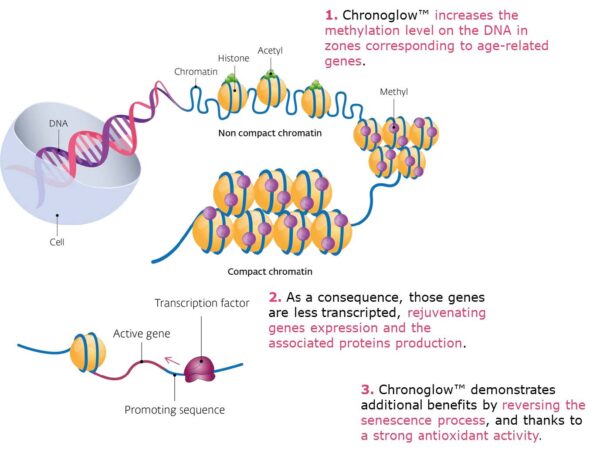
Because cryptic transcription emerges when chromatin control erodes, a methylome-first intervention complements UltraReverse’s DNMT3B-centric path. Together, they argue for a “fidelity stack”—stabilize the epigenome and directly suppress aberrant transcripts—to quiet noise at its source.
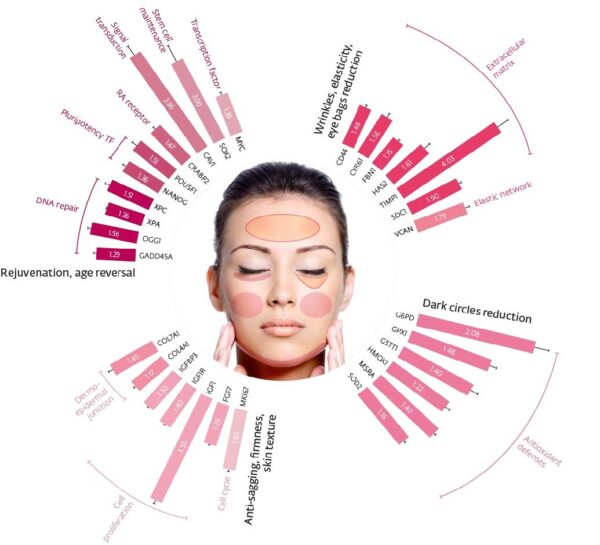
Building for Longevity with Less
The broader science is moving toward aging as loss of information—not metaphorically, but in the specific sense that methylation patterns, chromatin states, and repair capacities degrade, allowing spurious transcripts to proliferate. If cryptic transcription is a root cause of dysfunctional protein landscapes, then quieting that noise is a rational first principle for cosmetic longevity strategies. It shifts development from piling on more actives to calibrating the right ones that re-establish cellular code-keeping.
This approach is also operationally efficient. UltraReverse shows multi-hallmark coverage at tight doses; Chronoglow re-aligns the methylome with function; Sericoside executes dermal rebuilding—together enabling leaner INCI lines with broader benefits. Importantly, the same logic harmonizes with neuro-skin aging: healthier, better-regulated skin tissue is more likely to sustain the neuronal–epidermal interface that underlies tactile acuity, comfort, and reactivity with age.
Designing for Transcriptional Fidelity, Not Just Feel
Skin that reads its code correctly behaves younger—repair is swifter, proteins fold as intended, and structures hold. By treating cryptic transcription and epigenetic drift as first-order targets, formulators can move beyond incrementalism and design for cellular longevity. In practice, that means building around PrimalHyal™ UltraReverse to restore DNMT3B and suppress aberrant transcripts, pairing Chronoglow™ for methylome-to-proteome alignment, and deploying Sericoside to translate these corrections into ECM strength and visible resilience. The reward is a stable path to instrumented outcomes—without over-burdening your base.
On-Demand Session: The Hidden Code of Cellular Longevity—Givaudan’s first LinkedIn Live—covers neuro-skin aging, cryptic transcription, and the assay path to formulation-ready claims. Watch on demand:
Ready to formulate for transcriptional fidelity?
Contact your designated account manager or send us a request to discuss optimal use levels, phase placement, and compatibility testing for PrimalHyal™ UltraReverse, Chronoglow™, and Sericoside in fluid O/W, gel-cream, and sensitive-skin systems. We’ll help you design proof plans—from DNMT3B and PRO-seq to elasticity and eye-area endpoints—that stand up to scrutiny and deliver a cleaner signal of youth.
Resources
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. https://www.cell.com/cell/pdf/S0092-8674%2813%2900645-4.pdf
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2023). Hallmarks of aging: An expanding universe. Cell, 186(2), 243–278. https://www.cell.com/cell/pdf/s0092-8674%2822%2901377-0.pdf
- McCauley, B. S., et al. (2021). Altered chromatin states drive cryptic transcription in aging mammalian stem cells. Nature Aging. https://pmc.ncbi.nlm.nih.gov/articles/PMC8570536/
- McCauley, B. S., & E. (2022). Loosening chromatin and dysregulated transcription: A perspective on cryptic transcription during mammalian aging. Frontiers in Aging. https://pmc.ncbi.nlm.nih.gov/articles/PMC8789305/
- Saez, I., & Vilchez, D. (2014). The mechanistic links between proteasome activity, aging and age-related diseases. Current Genomics, 15(1), 38–51. https://pmc.ncbi.nlm.nih.gov/articles/PMC3958958/
- Frankowska, N., et al. (2022). Proteolysis dysfunction in the process of aging and age-related diseases. Frontiers in Aging. https://www.frontiersin.org/articles/10.3389/fragi.2022.927630/full
Citation note: This article integrates peer-reviewed, open-access literature to explain mechanisms (e.g., hallmarks of aging, chromatin drift, cryptic transcription, proteostasis decline) and uses supplier technical dossiers to document specific in-vitro/ex-vivo results for PrimalHyal™ UltraReverse, Chronoglow™, and Sericoside. Peer-reviewed sources provide independent biological context; supplier documents anchor the exact methods, models, and effect sizes for the featured ingredients—ensuring claims are mechanistically sound and experimentally traceable.









