Turning Omics Into Credible Skin Claims
Formulators are rightly skeptical of big promises. “Epigenetic rejuvenation.” “Proteome restoration.” These phrases can either clarify a mechanism or drift into marketing fog. The differentiator is evidence—and the way it is translated into cosmetic-appropriate, regulator-safe language. In the last decade, skin science has advanced far beyond single-endpoint assays. Multi-omic readouts—methylome, transcriptome, and proteome—now let us see how an active interacts with age-shifted biology and whether those shifts align with visible outcomes such as elasticity and radiance. The challenge is not the science; it is the wording.
In this article, we turn omics into a working claims workflow: what methylation and proteomic shifts actually mean in skin aging, how to align them with accepted guidance in the EU and U.S., and which clinical endpoints translate into visible, defensible results. We then apply that framework to a water-soluble botanical active with multi-omic data and placebo-controlled clinicals, showing how to write precise, review-safe language that still motivates the consumer.
From Mechanism to Message
Two friction points appear repeatedly. First, epigenetics is often invoked but seldom defined. In skin, age correlates with systematic shifts in DNA methylation patterns (CpG regions) and downstream gene expression impacting inflammation, barrier function, apoptosis, fibrosis, and extracellular matrix turnover. That is a legitimate scientific narrative, but cosmetic claims must stick to appearance/function of healthy skin—not treatment or prevention of disease. In the EU, claims must be supported, truthful, and fair under Regulation (EU) No 655/2013 and its Technical Document; in the U.S., FDA draws a firm line between cosmetics and drugs by intended use and claim type. If you reference “reversing aging,” you risk crossing into drug language. If you describe “improving the look of firmness and radiance, supported by clinical and omics data,” you are on safer ground.
Second, proteomic data can overwhelm. Age-related proteome damage—especially irreversible carbonylation—accumulates and correlates with functional decline in tissues, including skin. That does not grant license to claim medical prevention or repair of pathology. It does justify positioning an ingredient as helping “protect proteins from oxidative stressors” or “supporting proteins linked to barrier and elasticity,” provided you show the relevant in vitro/ex vivo endpoints and a clinical bridge. The literature supports proteostasis as a central aging axis and positions carbonylation as a robust oxidative stress biomarker, but your finished product needs reproducible instrumental outcomes to make appearance claims.
Chronoglow™: From Omics to Outcomes You Can Measure
Chronoglow™ was designed by biomimicry from Haberlea rhodopensis, a “resurrection” plant with coordinated gene-expression responses to stress. Givaudan’s controlled indoor cultivation and LED agronomy mitigate variability, ensuring consistent biomass and enabling greener sourcing. At the cellular level, the active demonstrated multi-omic effects: it modulated age-shifted methylation patterns in mature fibroblasts toward youthful profiles, activated gene sets linked to cellular defenses and ECM maintenance, and restored a portion of age-impacted skin proteins in explants. These omics signatures align with two macro benefits identified in the proteomic analysis: elasticity and complexion (barrier/anti-inflammatory/antioxidant).
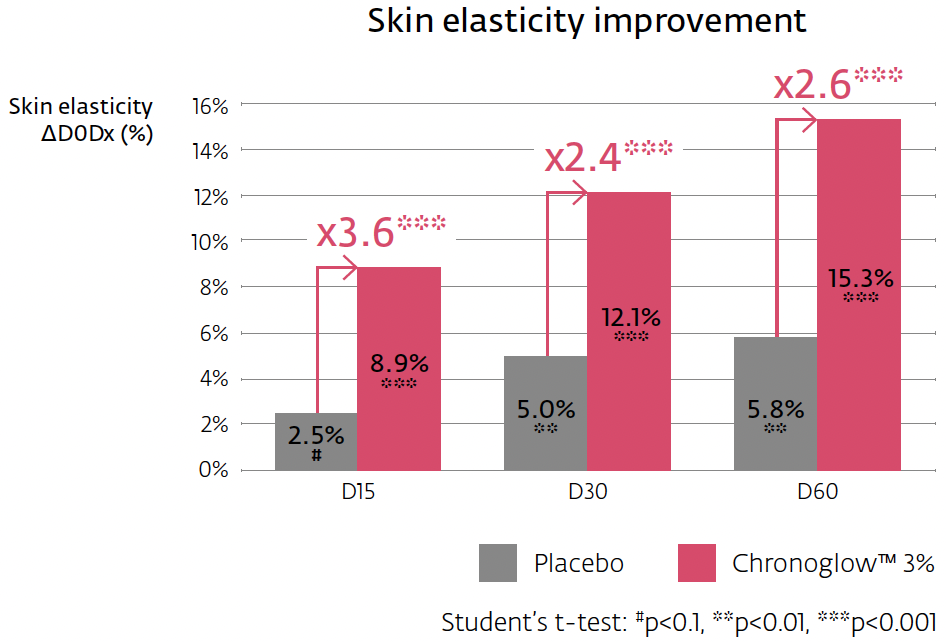
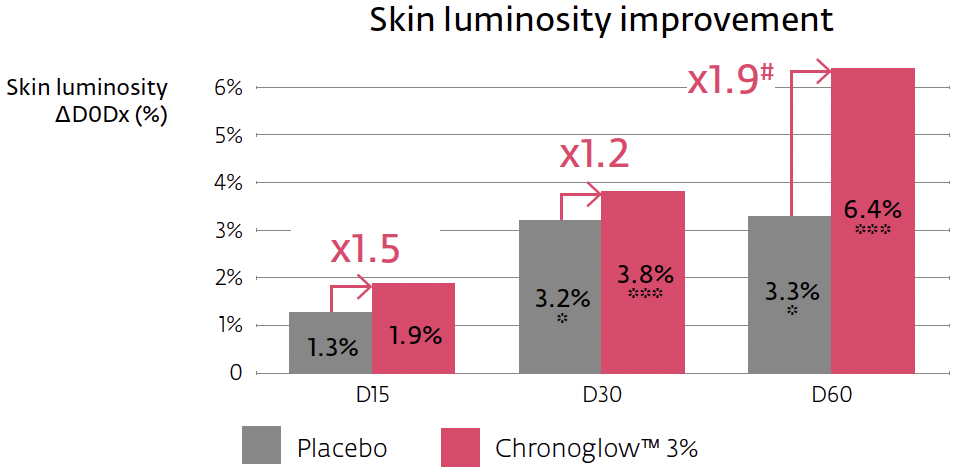
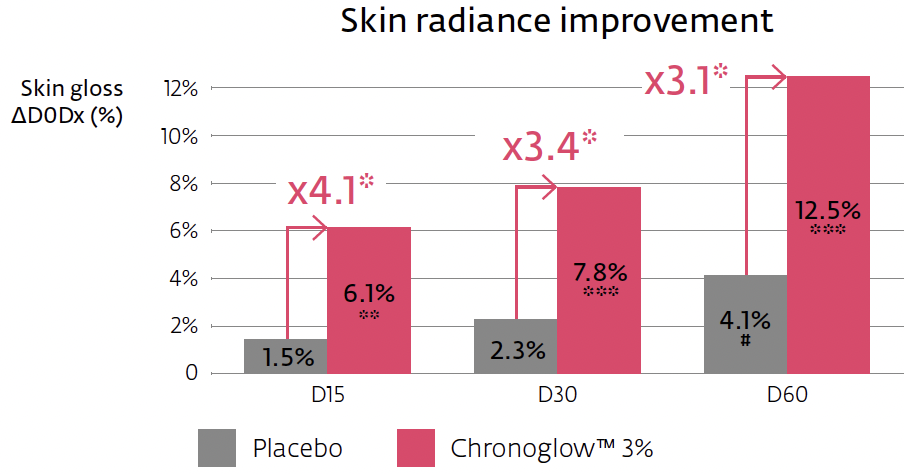
Crucially, the mechanism connects to clinically relevant outputs. In a 60-day hemi-face study (n=20, once daily), a cream with 3% Chronoglow™ improved overall elasticity (R2) significantly by two weeks, with continued gains through two months. Skin luminosity (L*, spectrophotometer) rose by week four and continued rising; skin gloss (perceived radiance proxy) improved as early as two weeks, again increasing through day 60. The protocol, instruments, and statistical thresholds are clearly specified, and the placebo-separated deltas are transparent—exactly the type of clinical bridge that makes multi-omic stories credible in the cosmetic context.
Omics That Deliver Reproducible Clinical Results
The methylome readout: In fibroblasts from a mature donor, Chronoglow™ shifted methylation at age-impacted CpG regions toward youthful conditions—an interpretation consistent with reviews that identify epigenetic drift as a hallmark of aging and a lever for age-associated gene expression. Translationally, this supports phrasing such as “helps counteract age-related epigenetic shifts associated with barrier and matrix integrity.” That phrasing remains descriptive, not therapeutic.
The proteome readout: In explants, 210 proteins differed significantly between young and mature skin. Treatment with Chronoglow™ brought 61 of those 210 age-impacted proteins (≈29%) toward the youthful state. Pathway analysis singled out keratinocyte migration and ECM preservation (elasticity) and barrier/anti-inflammatory/antioxidant functions (complexion). Within a claims file, that supports “helps preserve proteins linked to skin’s elasticity and complexion under oxidative stressors,” especially when combined with the in vitro antioxidant/anti-senescence findings and the clinical improvements in R2, L*, and gloss.
Stress-response and senescence: Chronoglow™ reduced SA-β-gal activity in senescent fibroblasts by ~31% and showed up to ~90% protection in oxidative assays while lowering oxidized proteins ex vivo—mechanistically consistent with the broader proteostasis literature on carbonyl damage. In compliant language, this translates to “helps reduce markers associated with cellular senescence in vitro and helps protect proteins from oxidative stress in skin models,” followed by the clinical bridge to appearance outcomes.
Clinical bridge and instrumentation: The elasticity measurement (Cutometer® R2) captures the skin’s ability to return to rest position after deformation; L* captures brightness; gloss reflects specular radiance. At 3% Chronoglow™, R2 improved by up to ~15.3% at day 60 (significantly > placebo, ≈2.6×), with a detectable +8.9% already at two weeks. L* improved up to ~6.4% at two months (~1.9× vs. placebo), and gloss rose by up to ~12.5% at two months, detectable by week two and >4× placebo early on. Because these are instrumented, placebo-controlled deltas, you can credibly say “visibly firms and boosts radiance,” while specifying the endpoints in technical materials.
Formulation pragmatics: Chronoglow™ is water-soluble, used at 1–3% (3% in the clinical), and added to the water phase under 40 °C. The active itself is preservative-free; design the finished system with appropriate hurdles. These conditions reduce activity drag risks and simplify integration into O/W emulsions or gel-creams—useful if pairing with exfoliating acids, retinoids, or niacinamide to stack near-term radiance with long-term tone uniformity, while monitoring pH and irritation potential.
A Playbook for Compliant Omics-Backed Claims
The EU Technical Document on Cosmetic Claims expects a coherent line from evidence to message: appropriate testing for the claim, sound methodology, and fair presentation. In practice, that means aligning omics endpoints to clinically perceptible outputs and avoiding medicinal language. In the U.S., the FDA’s “intended use” principle demands that you speak to appearance and cosmetic benefits, not structure/function in a medical sense. The safest path forward is a claims architecture where mechanism explains how the cosmetic appearance changes are supported—not a promise to treat or reverse disease.
A disciplined mapping looks like this:
Omics signal → Biological pathway → Instrumental endpoint → Consumer-facing claim. For Chronoglow™, methylome and proteome shifts point to barrier/matrix support and antioxidant defense; these translate into improved R2 elasticity, higher L*, and increased gloss in clinical settings; consumer-safe language becomes “helps improve the look of firmness and radiance in two weeks, with continued improvement over two months,” anchored by exact numerical deltas in technical sheets.
Finally, the proteostasis lens is not a fleeting trend. Reviews argue that protecting the proteome is a rational strategy for mitigating visible skin aging because carbonylation and other irreversible modifications accumulate and impair function. Ingredients that demonstrate protein-level effects in human skin models—and then show clinical appearance changes—will set a higher bar for substantiation. Done correctly, omics becomes a clarity tool rather than a buzzword.
From Findings to Formulas: What the Data Supports—and How to Use It
Epigenetic and proteomic data can elevate cosmetic claims—if you keep them tethered to visible outcomes and regulator-safe language. Chronoglow™ provides a useful case study: multi-omic modulation aligned to elasticity and complexion pathways, in vitro and ex vivo stress-response support, and a placebo-controlled clinical bridge demonstrating improvements in R2, L*, and gloss on realistic timelines. The formulation work is straightforward, the endpoints are reproducible, and the claims can be written cleanly without overreach.
Looking to translate this evidence into claim-ready specs for your next launch?
For ingredient documentation, formulation guidance, or to discuss study design for substantiation, submit a request on Deveraux Specialties’ website or contact your dedicated sales manager for support.
Resources
- Dal Pozzo, L., et al. (2024). Role of epigenetics in the regulation of skin aging and rejuvenation. Pathologie Biologie. https://www.sciencedirect.com/science/article/pii/S0753332224004761
- Pereira, B., et al. (2024). Epigenetic reprogramming as a key to reverse ageing and rejuvenate cells. Ageing Research Reviews. https://www.sciencedirect.com/science/article/pii/S1568163724000229
- He, J., et al. (2022). Application of omics technologies in dermatological research and skin care product development. Skin Research and Technology. https://pubmed.ncbi.nlm.nih.gov/33759323/
- Mikhed, Y., et al. (2024). Protecting the proteome may be the key to preventing skin aging. Mechanisms of Ageing and Development. https://pubmed.ncbi.nlm.nih.gov/39193671/
- European Commission. (2017). Technical document on cosmetic claims: Compliance with Regulation (EU) No 655/2013 (Guidance). https://ec.europa.eu/docsroom/documents/24847/attachments/1/translations/en/renditions/native
- U.S. Food & Drug Administration. (2024). Is It a Cosmetic, a Drug, or Both? (Or Is It Soap?) https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap
(Note: These sources were chosen to pair primary, ingredient-specific evidence with independent scientific and regulatory context so claim language stays accurate and compliant. The Chronoglow™ product literature supplies hard facts—INCI, use level, processing—and the exact multi-omic and clinical endpoints (R2 elasticity, L*, gloss) used in our examples. EU claims guidance (Reg. 655/2013 Technical Document) and the FDA’s “cosmetic vs. drug” criteria anchor wording to current review standards, preventing therapeutic overreach. Recent peer-reviewed reviews on skin epigenetics explain why methylation shifts matter biologically, proteome/carbonylation papers justify statements about protecting protein integrity under oxidative stress, and dermatology “omics in R&D” overviews provide accepted methods for linking pathway-level signals to instrumented outcomes. Together, they create a defensible chain of evidence—from mechanism to measurement—that formulators can reproduce in real products.)









